Slack Connect lets two companies move as quickly as one. Hatch a partnership. Build something new. Learn more about Slack Connect. And you can chat face to face, with just a click. And video calling, too. In short: it’s a more human way to work. Thanks to Slack Help center, I'm able to set a reminder on specific days but not an all week days. My question is: How to set a single reminder on all week day? Something like this: /remind @channel 'It's time for daily stand up!' Monday Tuesday Wednesday Thursday Friday at 11:45am. I found a workaround but it's not really satisfying: set one reminder each day. It's time to stop forgetting. Receive regular grouped messages for daily, weekly or monthly routines. Once setup our chat bot will send you notifications to ensure that your tasks are completed and logged over time. Reminder Bot's features are free for 28 days and are extended via small monthly payments. You can snooze the reminder for 20 minutes, 1 hour, or until the next day at 9 a.m. Mark a reminder as complete. To mark a reminder as complete, select Complete in the reminder message from Slackbot, or from the list displayed by the /remind list slash command. Click View completed reminders to view items you’ve marked as done. Slack daily reminder software. Every day at a given time, a message is posted in a team channel with a reminder and instructions for posting a daily status update. Mentioning a team’s user group will notify everyone.
The Strong Nuclear Force is fine tuned for life. If the strong force was weaker than it is, the chemical elements needed for life would not be stable, and we would not be here. If it were stronger, all the hydrogen in the universe would have been burned to helium in the Big Bang. As a result, there would be no long-lived stars like the sun, and no water. There would probably be no complicated chemistry in the universe, and we would not be here.
Thus, the strong nuclear force is a very short-range force. The line for the strong nuclear force cuts the distance axis at about 0.5 fm and approaches it very closely at about 3.0 fm. If asked to sketch this graph in an examination, make sure that you carefully label the axes and make the graph line approximately correct. Scientists are aware of four fundamental forces- gravity, electromagnetism, and the strong and weak nuclear forces. Most people have at least some familiari. Strong force: This is a short-range attractive force. 'Short range' refers to the fact that the force dies off exponentially in distance. This means that a nucleon is only affected by the strong force of its nearest neighbors. However, within that distance of less than 2.0 E-15 m, it overwhelms the other forces. The strong force is the most powerful of all the known forces. It is roughly 137 times stronger than the electric force. It is the force that holds quarks together to form the proton and neutron (nucleons), and its residual nuclear force holds nucleons together in an atom’s nucleus to form atoms.
(A slightly longer version of this article, which includes some extended quotations from the source literature, and discusses one important objection to the fine tuning of the strong nuclear force, can be found at www.focus.org.uk/strongforce_long.pdf)
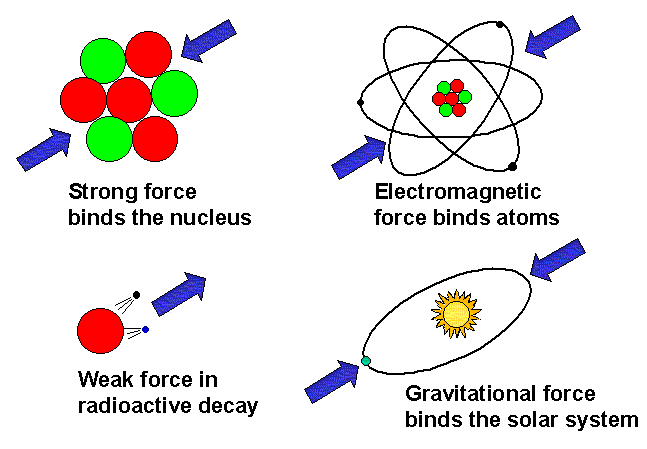
In everyday life we’re only aware of two fundamental forces: gravity and electromagnetism. Physicists know about two more forces, which only work at very short range (inside atoms): the strong nuclear force and the weak nuclear force.
This article is about the strong nuclear force – the force that holds protons and neutrons together in the nucleus of atoms. It is about ten thousand billion billion billion billion times (1040) times more powerful than the force of gravity.
Picture two protons. They are pulled together by the strong nuclear force (as long as they are within range to start with.) But the electromagnetic force pushes them away from each other, because they both have the same positive electric charge.
The electromagnetic repulsion wins over the strong nuclear force attraction, and you can’t get two protons to stick together. So a nucleus made of just two protons (called a ‘diproton’) isn’t stable.
But if you add a neutron the balance of the forces shifts: neutrons feel the strong nuclear force, but they don’t feel the electromagnetic force, because they’re electrically neutral. So adding a neutron is enough to tip the balance: a nucleus made of two protons and one neutron is stable.

So the balance between the strong nuclear force and the electromagnetic force affects the way protons and neutrons can combine to make stable atomic nuclei. This balance has to be fine-tuned for life to be possible.
What would happen if the strong nuclear force were a bit weaker?
If the strong force were a bit weaker, it would not be able to hold atomic nuclei together against the repulsion of the electromagnetic force. According to Barrow and Tipler:
Strong Nuclear Force Range
‘A 50% decrease in the strength of the nuclear force… would adversely affect the stability of all the elements essential to living organisms and biological systems.’[1]

A bit more of a decrease, and there wouldn’t be any stable elements except hydrogen.
What would happen if the strong nuclear force were a bit stronger?
If the strong nuclear force was just a bit stronger compared to the electromagnetic force, two protons could stick together in spite of their electromagnetic repulsion (forming a diproton).
If this happened, all the hydrogen in the universe would have been burned to helium in the big bang. It’s very difficult to imagine how a universe with no hydrogen could produce the complicated chemistry needed for life– there would be no water, for a start, and there would be no long-lived stars like the sun. (Stars made from helium burn up much more quickly than stars made from hydrogen.) Barrow and Tipler again:
‘All the hydrogen in the Universe would be burned to He2 during the early stages of the big bang and no hydrogen compounds or long-lived stable stars would exist today.’[2]
Conclusion
If the strong nuclear force was weaker than it is, the chemical elements needed for life would not be stable, and we would not be here. If it were stronger, all the hydrogen in the universe would have been burned to helium in the Big Bang. As a result, there would be no long-lived stars like the sun, and no water. There would probably be no complicated chemistry in the universe, and we would not be here.
David Couchman MA, M.Sc, M.Min, August 2010
[1] Barrow, J D and Tipler, F J, ‘The Anthropic Cosmological Principle’ Oxford University Press 1986, p. 327
[2] Barrow and Tipler, p. 322
| The strong nuclear force holds together the protons and neutrons in the nucleus of an atom. This is actually a side effect of its function binding quarks together to make the protons and neutrons themselves. The particle of the strong force is called the gluon because of the strong force’s glue-like properties. |
The strongest of the subatomic forces is the aptly named strong nuclear force. In the realm in which it operates, it is about 100 times stronger than the next-strongest force (electromagnetism). But it isn’t just its strength that distinguishes it from the other forces. It has other properties that differ from, for instance, the features of a magnet. The force between two magnets extends over a long distance and becomes stronger as the magnets are brought closer to one another. In contrast, the strong nuclear force is a lot more like glue. If you have two marbles made sticky by some kind of adhesive, they will cling together when they are made to touch each other. However, once the two marbles are separated by even a very small distance, they no longer feel any attractive force at all.
In homage to the force’s commonalities with how glue behaves, the force-carrying particle for the strong force is called the gluon. Gluons are responsible for binding protons and neutrons together inside the nucleus of an atom. This is crucial for building atoms, but this nuclear binding is actually a side effect of what the gluon really does—hold together the quarks that make up protons and neutrons. In high-energy physics experiments, it is the quark-quark binding that is of the greatest interest.
The distance over which the nuclear force is active is about 1 femtometer (10-15 or one quadrillionth of a meter). To give an idea of just how mind-bogglingly small that is, if a proton were as thick as a sheet of paper, by comparison you’d be so big that, if you stood on the Earth, your head would touch the Sun.
In the last Nutshell, we were introduced to the photon, the quantum of the electromagnetic force. Because the photon is electrically neutral—that is, it has no electric charge—photons don’t interact with each other. In contrast, every gluon has a strong nuclear charge. Thus gluons interact not only with quarks, but also with other gluons. This gluon self-interaction property is one of the reasons that the strong force acts like glue instead of magnets.
The charge of the nuclear strong force is known as color. In a subatomic context, three colors—red, blue and green—are carried by the three quarks in a proton, resulting in a simple color scheme. In contrast, the force-carrying gluons have a rather complex color palette, one with a mix of both color (the charge carried by quarks) and anticolor (the charge carried by antiquarks). In total, there are eight different color combinations that gluons can carry. (If you’re wondering why three colors and three anticolors combine to make eight gluons and not nine, the answer can be found here.)
Gluons were discovered at the German laboratory DESY in the late 1970s. They play a key role in many of the studies performed at the Tevatron and the LHC. Drivers remotecontrol network & wireless cards.
Strong Nuclear Force Equation
—Don Lincoln
Strong Nuclear Force Meaning
Want a phrase defined? Have a question? E-mail today@fnal.gov.
