How to Find the Number of Protons, Neutrons, and Electrons. Step by Step Explanation with Examples
- Mass Number Atomic Number And Isotopes
- Mass Number Atomic Number
- Notation Mass Number Mass Number Atomic Number
In this post, we’ll be going over how to determine the number of protons, neutrons, and electrons in an atom or ion.
So, we can say that the atomic mass of an element is the number of times its atom is heavier than 1/12 th of the mass of an atom of C-12, e.g., the atomic mass of Mg is 24 u. It means the atomic mass or mass of one atom of Magnesium is 24 times more than the 1/12 of the atomic mass of carbon 12. The number of protons in the nucleus is called the atomic number. The atomic number of each element is unique. The combined number of protons and neutrons in an atom is called the atomic mass number. While the atomic number always stays the same some elements have atoms with different atomic mass numbers.
We’ll first start by discussing what each of the components in the nuclide notation means. Then we’ll go through two examples together.
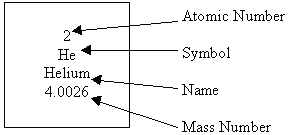
The Nuclide Notation
The letter(s) in the middle is the symbol of the element.
The number on the bottom left corner is the atomic number, which tells you the number of protons.
The number on the upper left corner is the mass number, which is equal to the neutrons and protons added together.
Lastly, the charge is on the upper right corner. If there isn’t any number or signs, then it means that atom has no charge and is neutral.
Rules to Finding Number of Protons, Neutrons, and Electrons
# of protons = atomic number
# of neutrons = mass number – atomic number
# of electrons = atomic number – charge
That’s it!
Examples
Great, lets apply the rules to some examples.
# of protons = 17
# of neutrons = 37 – 17 = 20
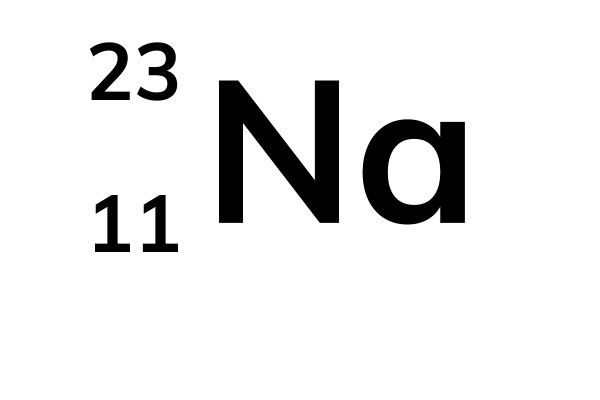
# of electrons = 17 – 0 = 17
# of protons = 16 (the atomic number is not given, but can be found on the periodic table)
# of neutrons = 32 – 16 = 16

# of electrons = 16 – (-2) = 18
Additional Practice
Try these on your own and check the answer below
- 78Se2-
- 39K+
ANSWERS
- 34 protons, 44 neutrons, 36 electron
- 19 protons, 20 neutrons, 18 electron
Additional Resources
Mass Number Atomic Number And Isotopes
If you have any questions, leave a comment below.
Mass Number Atomic Number
Atomic Number and Mass Number
When you study the periodic table, the first thing that you may notice is the number that lies above the symbol. This number is known as the atomic number, which identifies the number of protons in the nucleus of ALL atoms in a given element.
The symbol for the atomic number is designated with the letter Z. For example, the atomic number (z) for sodium (Na) is 11. Drivers ploytec. That means that all sodium atoms have 11 protons. If you change the atomic number to 12, you are no longer dealing with sodium atoms, but magnesium atoms. Hence, the atomic number defines the element in question.
Recall that the nuclei of most atoms contain neutrons as well as protons. Unlike protons, the number of neutrons is not absolutely fixed for most elements. Atoms that have the same number of protons, and hence the same atomic number, but different numbers of neutrons are called isotopes. All isotopes of an element have the same number of protons and electrons, which means they exhibit the same chemical behavior. Because different isotopes of the same element haves different number of neutrons, each of these isotopes will have a different mass number(A), which is the sum of the number of protons and the number of neutrons in the nucleus of an atom.
Mass Number(A) = Number of Protons + Number of Neutrons
Notation Mass Number Mass Number Atomic Number
The element carbon (C) has an atomic number of 6, which means that all neutral carbon atoms contain 6 protons and 6 electrons. In a typical sample of carbon-containing material, 98.89% of the carbon atoms also contain 6 neutrons, so each has a mass number of 12. An isotope of any element can be uniquely represented as AZX, where X is the atomic symbol of the element, A is the mass number and Z is the atomic number. The isotope of carbon that has 6 neutrons is therefore 126C. The subscript indicating the atomic number is actually redundant because the atomic symbol already uniquely specifies Z. Consequently, it is more often written as 12C, which is read as “carbon-12.” Nevertheless, the value of Z is commonly included in the notation for nuclear reactions because these reactions involve changes in Z.
